Microalgae downstream
Introduction
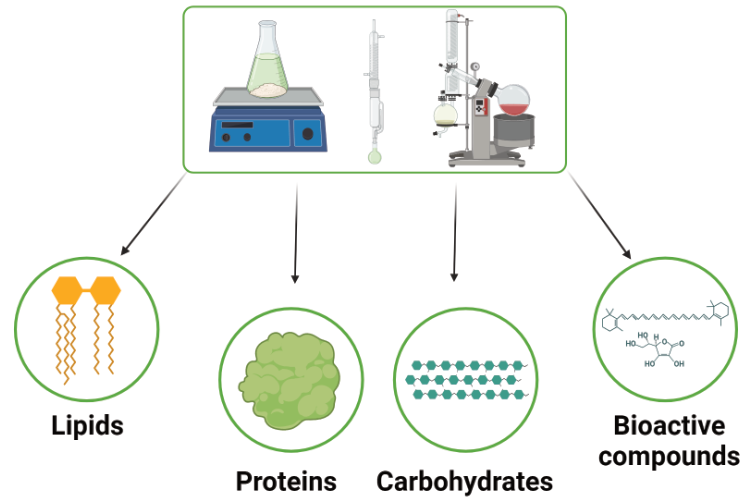
After microalgae cultivation is complete, the produced biomass needs to be collected, and the desired compounds and compounds must be extracted and separated. Additionally, a pretreatment method is typically applied to disrupt the microalgae cells to enhance the recovery of the intracellular components. These steps of harvesting, pretreatment, and extraction collectively form what is known as downstream processing.
1. Harvesting
A typical downstream process begins by ‘harvesting’ the biomass. The microalgae are cultivated in very dilute conditions, usually at 0.5 g L-1 solids and the large water volumes make impractical further processing. There are different techniques to remove the excess water although no one has emerged as clearly superior. Usually a combination of technologies is applied, first a ‘harvesting’ step for the formation of a slurry of 1-5 % wt followed by a ‘dewatering’ step to reach the final highly concentrated paste. These techniques can be mechanical, chemical, biological or electrical. Three main techniques with prospects for upscaling are centrifugation, membrane separation and flocculation.
Centrifugation is a very common technique already applied in other industrial applications. It offers noteworthy advantages such as high efficiencies, fast outcomes and no addition of chemicals. This comes at the cost of high initial capital investments as well as energy consumption. The latter is dependent on the technical characteristics of the centrifuge. The efficiency of the centrifugation is species-related since it is related to the cell size and their density difference to the culture medium.
In membrane separation, microalgae culture passes through filters operating under gravity, pressure or vacuum force to hold back the biomass in a thick paste form. The system can operate either in continuous or batch mode. This approach has the advantages of delivering undamaged biomass and lack of chemical addition. A number of materials (such as polyether sulfone, polyvinylidene fluoride, polytetrafluoroethylene and others) have been tested along with various conditions (microfiltration:0.1–10 μm, microfiltration:10 μm, ultrafiltration: 0.02–0.2 μm etc). The challenges usually associated with these methods include relatively low capacities and rapid fouling of microalgae secretions of organic matter.
During flocculation, the microalgae aggregate through surface charge neutralization, electrostatic patching or bridging after addition of flocculants. The formed agglomerates then separate through simple gravity-induced settling. It has been proposed as a potentially superior technique for harvesting microalgae as it can be used for large scale with a wide range of microalgae species. Flocculation can be either chemical, auto-flocculation or bioflocculation, depending on the nature of the flocculant that is added. Drawbacks with this method is the relatively high energy cost, the potential flocculant toxicity, or non-feasibility of scaling up.
2. Pretreatment techniques
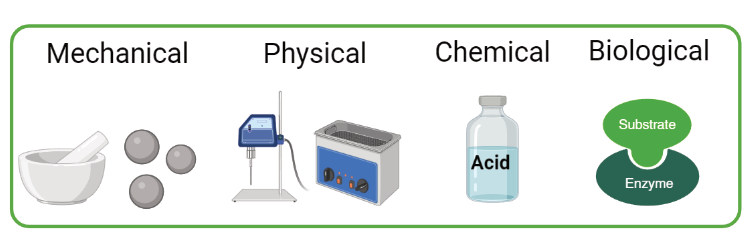
The characteristic resistance of microalgae cells to intracellular extraction is a significant challenge in the microalgae downstream processing. This is commonly attributed to the rigid cell walls that surround the cell which form a barrier preventing interaction between intracellular molecules and external solvents. To counter this, a pretreatment method is usually necessary in order to rupture the cells and enhance the accessibility to the targeted molecules. This treatment can be physical (mechanical, thermal, electrical, etc), chemical, biological or a combination of the above. An optimal cell disruption technique should operate on wet biomass, be energy efficient and suitable for large scale industrial applications. It is additionally crucial that the applied method will not contaminate or destroy any of the desired compounds.
Physical pretreatment methods include diverse techniques that focus either on the entire destruction of the cell such as bead mill or high-pressure homogenizer (HPH) or milder ones that leave the overall cell structure intact such as microwave treatment.
Bead milling is a technique used routinely for disruption of microalgae and other microorganisms. During milling, biomass is grinded against solid metallic surfaces (beads) under agitation at high frequencies, resulting in the breaking of the cell wall and the total destruction of the cell. It is highly effective for total disruption of cells although it can face scaling challenges in industrial applications and is considered very energy intensive process.
High pressure homogenization (HPH) is another technique aiming to the destruction of the entire cell structure. During operation, the cells are broken up due to high shear stress when forced to flow through a small orifice under high pressures. The end result is an emulsion of proteins, lipids and other cell fragments. The typical range of working pressures is 100-150 MPa with multiple passes required through the homogenizer although, extreme pressures (such as 300 MPa) might be applied to avoid this. A temperature increase of about 2 °C for every 10 MPa of applied pressure is reported which means that a cooling system should be implemented for heat sensitive products such as proteins or pigments. HPH is a well-established technology with a long history in the dairy industry. In order to counter its high energy demand, treatment should take place in high biomass concentrations and ideally with cells not equipped with strong cell walls. However, the resulting emulsion of the different microalgae cell components could pose a significant barrier for a cascade extraction in a biorefinery.
During ultrasound pre-treatment or ultrasonication, acoustic waves of 20 kHz and higher are applied to the microalgae suspension. Disruption is achieved through different pathways, one of which is through cavitation. The applied ultrasound leads to the formulation of microbubbles which expand and contract continuously until they implode disrupting thus the cells. Other direct effects occurring at low power and not involving cavitation, include radiation force and acoustic steaming. Challenges with the implementation of this technique include the temperature rise of the suspension during application and the production of free radicals and side chemical reactions with an uncertain effect on the final product. The exact disruption mechanism is also relatively unexplained with more research work required.
Microwave treatment has also been examined as a pretreatment method. Microwaves are defined as short waves of electromagnetic energy ranging from 300 MHz to 300 GHz and they function by supplying heat to the microalgae biomass which leads to cell rupture. Interestingly, microwave technology has been applied not only as a pre-treatment method but additionally during the actual lipid extraction in order to enhance it. In literature, such techniques are distinguished as ‘microwave assisted extraction’. The major challenges of upscaling microwave treatment would be the limited penetration depth of the microwaves into the absorbing medium and some concerns regarding the energy requirements.
The main technique in the chemical pretreatment methods is acid/base hydrolysis of the cells. This methodology is particular effective for the production of bio-ethanol from microalgae since the complex cell wall carbohydrates are released and broken down to monosugars with the addition of simple acids such as hydrochloric or sulphuric acid. The disadvantage of these techniques is the volumes of chemicals required as well the fact that the involved extreme conditions, such as high temperatures, might damage other valuable compounds.
Biological pre-treatment involves the addition of a single or several enzymes, specially selected for each microalgae. These enzymes proceed then to digest the cell wall while leaving the intracellular components intact. Typical enzymes include cellulase, protease, lysozyme and others. It is an attractive methodology due to the mild operational conditions (temperature, pressure etc.) and low energy requirements. It is also commonly paired with other techniques such as microwaves in order to reduce costs. The main bottleneck however, is the prohibitive expense of these enzymes at industrial scale and from a more technical point of view, the determination of the cell wall composition of the desired microalgae in order to design the appropriate enzyme mix.
3. Extraction of intracellular components
A number of valuable compounds can be produced and be extracted from microalgae. The ones that are produced in largest quantities include proteins, lipids and carbohydrates. Due to limited industrial applications of microalgae yet, most of the developed extraction techniques are still limited to pilot level demonstrations however. The selection of the appropriate extraction method depends firstly on the targeted molecule (water cannot normally be used for hydrophobic lipids as a solvent for example) and by the final usage of the product.
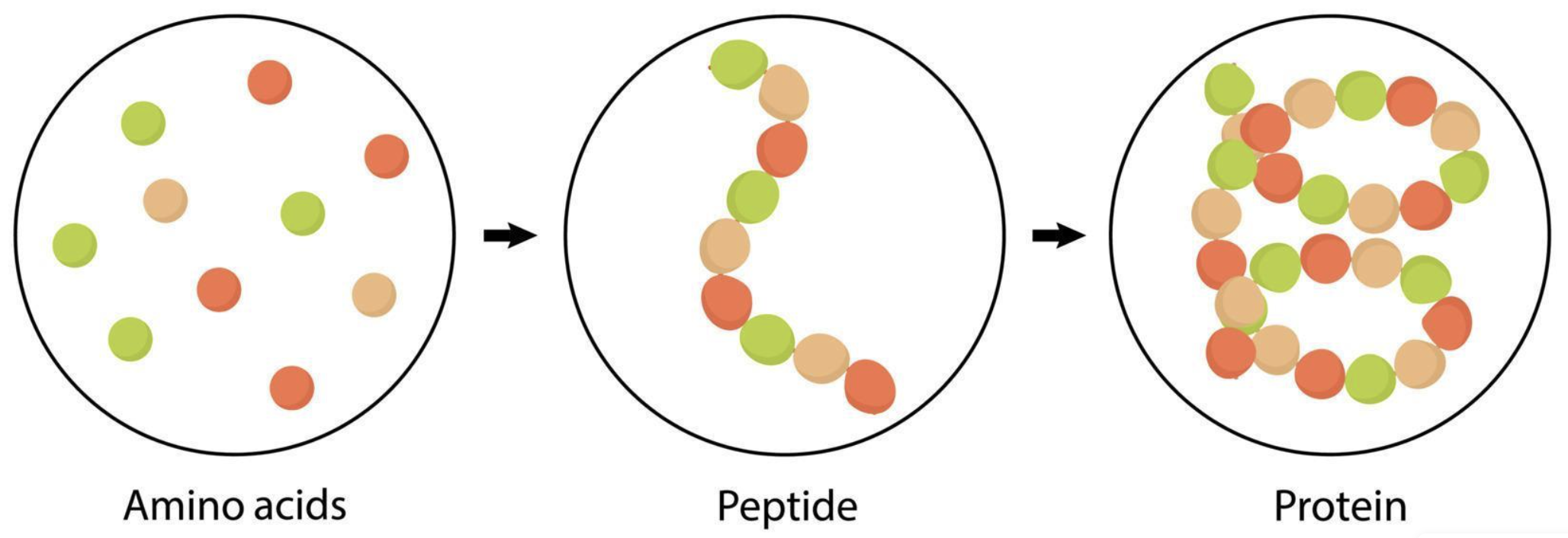
Proteins
Proteins are large biomolecules composed of amino acids that are crucial for the metabolism’s proper function. Amino acids are organic compounds with a structure of an amine (-NH2), carboxyl (COOH) and a side chain R. A short chain of 20-30 amino acids linked by peptide bonds is a peptide. A longer chain is called polypeptide or protein. Microalgae can exhibit high protein content and are capable of producing all essential amino acids and are under consideration for human consumption. Protein extraction is typically performed using aqueous, acidic or alkaline methods and the removed protein molecules are separated afterwards through centrifugation, ultrafiltration, precipitation or chromatography. Due to concerns for the bioavailability of the proteins, a common strategy to improve their digestibility is to hydrolyze them into amino acids. This hydrolysis can be either chemical or enzymatic in nature. Industrial scale protein extraction from microalgae is rarely studied. Common challenges with upscaling include the multiple differences between microalgal species, variation in the cell structure, variation in the intracellular protein content and release of protein degrading (protease) enzymes from the cells.
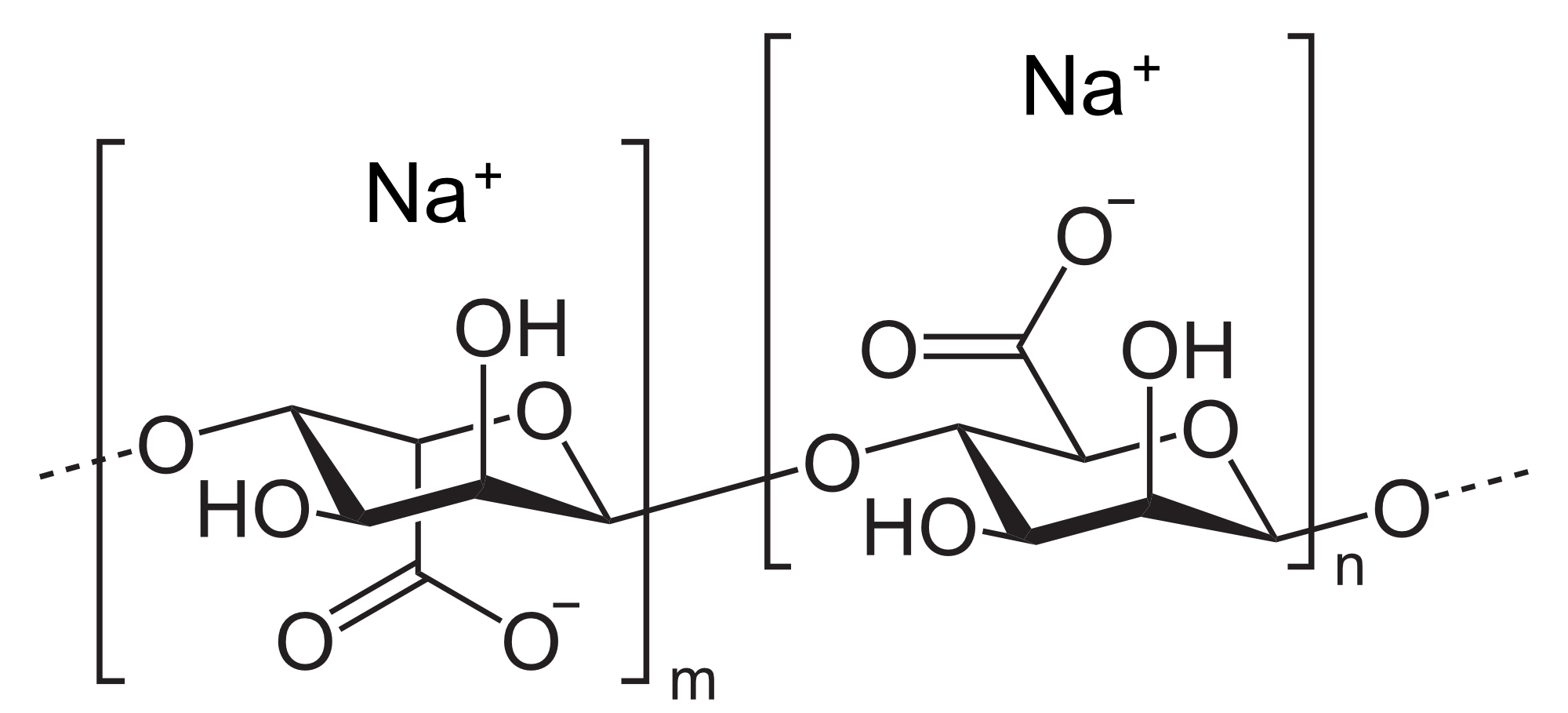
Carbohydrates
Carbohydrates also known as polysaccharides or sugars are composed mainly of carbon, hydrogen and oxygen. The smaller carbohydrate molecules, such as glucose, are called monosaccharides. Similar to the peptides and polypeptides, longer chains of monosaccharides linked together are known as polysaccharides, and they are mainly encountered as starch. Carbohydrates play an essential role in microalgae processing since they are one of the significant components of their formidable cell walls. Commercially extracted polysaccharides from algae include mainly alginates, agar and carrageenan. They are also studied for the production of biofuels, primarily bioethanol and biomethane (biogas) or increasingly in the bioplastics sector. For analytical purposes, the carbohydrates are typically undergoing acidic hydrolysis according to the National Renewable Energy Laboratory (NREL) procedure. Afterwards, they can be quantified using colorimetric methods like the phenol-sulfuric acid or anthrone.
For the production of bioethanol, the microalgae biomass is pretreated and subsequently hydrolyzed with the addition of enzymes or other microorganisms with the yeast Saccharomyce being a very common one. Hydrolysis can be either enzymatic or acidic at temperatures ranging between 120- 140 °C for 15-30 min with glucose being the monosaccharide most frequently produced. These simple sugars can then be fermented into bioethanol. The carbohydrates can also be used as feedstock for biogas. As the name implies, it is a gaseous biofuel composed mainly by methanol and is produced by anaerobic digestion. Microalgae derived biogas has the advantage of low sulfur content however, the C/N ratio of the feedstock, an important parameter for success of the anaerobic digestion, is relatively low.
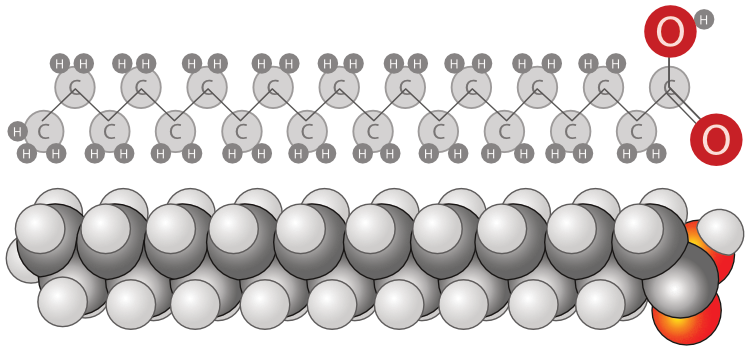
Lipids
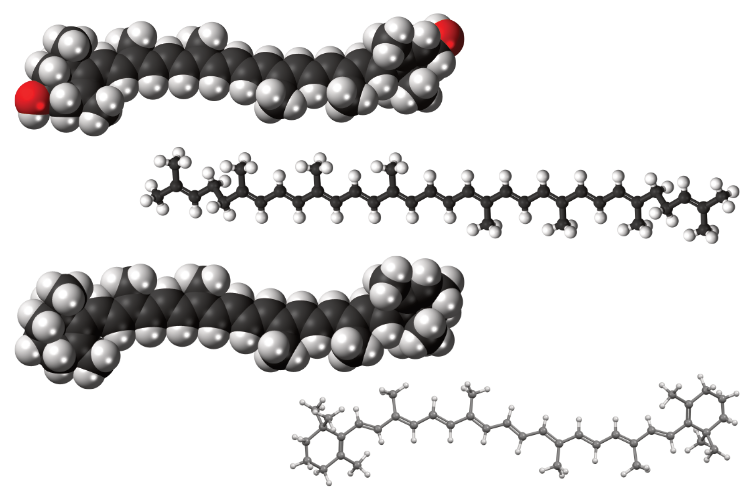
Lipids are a large family of organic compounds encountered naturally in liquid and solid phases. Their main constituent is typically glycerides and are characterized by their very low solubility in water. Glycerides or acylglycerols are esters formed from glycerol and fatty acids. Fatty acids are large molecules with a hydrocarbon chain and a carboxylate (ROO-) at their head. Lipids produced by microalgae are neutral (free fatty acids and triglycerides) and polar, which in turn are subdivided into phospholipids (fatty acids with a phosphate group) and glycolipids (fatty acids with an oligosaccharide group). Microalgae lipids are an excellent feedstock for biodiesel products. Certain microalgae species can also produce significant polyunsaturated fatty acids (PUFAs) with a recognized nutritional value. Microalgae lipid extraction has been extensively studied due to its high potential as a renewable biodiesel source.
Industry often uses classical organic solvent extraction techniques like soaking, counter-current, or pressurized liquid extraction. Lipid extraction with organic solvents is the most conventional and examined method to date. For total lipid extraction from microalgae, a ‘co-solvent mixture’ of polar and neutral solvents is usually required. The co-solvent could form a single phase (monophasic extraction) or two phases (biphasic extraction).
Generally, an ‘ideal’ solvent has to fulfill multiple criteria. Apart from compatible polarity, a solvent has to be easy to recycle, nontoxic or harmful to the environment or the end product, the price of bulk solvent and its miscibility with water in case of wet extraction. A number of organic solvents have been examined. Chloroform:methanol is limited to bench-scale applications but is considered a gold standard for lipid estimation. Other common choices are hexane, heptane, ethyl acetate, acetone, ethanol and isopropanol. Hexane and ethanol or acetone are popular choices, due to the fact that their use is already accepted in food processing and hexane’s highly neutral nature and low boiling point. Hexane is in fact already applied industrially for extraction of vegetable oil from oil seed. Consideration should also be given on the emerging trend of Green Chemistry. These so called ‘bio-solvents’ are derived from sustainable sources such as agricultural wastes and not from the petrochemical industry like hexane. 2-Methyltetrahydrofuran (2-MeTHF), Cyclopentyl methyl ether (CPME) or various terpenes such as d-limonene have already been tested successfully and in theory could replace the more conventional solvents.
Other forms of environmentally lipid extraction is the utilization of supercritical carbon dioxide (sCO2). Carbon dioxide is selected due to its inert, nontoxic nature and its almost ambient critical temperature. At relatively modest conditions (7.4 MPa and 31.1 °C), carbon dioxide is above its critical point, with little distinction between physical phases, resulting in a fluid-like density and gas viscosity and diffusivity. At this state, mostly neutral lipids are highly soluble in scCO2 and to some extent polar as well. Once extraction is complete, then a release of pressure, returns CO2 to its gaseous state, leaving the extracted oil behind to be collected. This method is highly promising due to the high selectivity it presents for lipid extraction compared to other components and easy solvent separability. However, it faces high equipment costs and energy usage requirements. Moreover, the affinity to mainly neutral lipids, means that it is either inappropriate for polar lipid extraction or will need to be supplemented with an organic solvent such as ethanol.
Carotenoids
Carotenoids are a diverse group of various natural pigments. They are responsible for the yellow, orange and other colors encountered in land plants or animals. They are greatly valued both as color additive to food as well as nutrition supplements due to their reported antioxidant function in the prevention of various diseases. Microalgae are noted for their high output of carotenoids, whose ‘natural’ productivity is considered much higher than synthetic ones. carotenoids are a class of lipophilic terpenoid pigments with a 40-carbon chain of alternating double and single bonds (polyene). Carotenoids are divided into two groups: the carotenes (β-carotene and lycopene) which are pure hydrocarbons and xanthophylls (lutein, astaxanthin etc) that contain oxygen in their structure. Being highly hydrophobic, carotenoids cannot be extracted with water. Their extraction methods therefore share a lot of similarities with the lipid extraction ones. They should be handled with special care and at inert conditions however, due to their highly oxidative nature to prevent their degradation.
Biorefineries
In order for the microalgae to break away from limited niche markets and reach more widespread commercialization, the idea of a single product needs to be phased out and be replaced with the biorefinery scheme. This concept, much like the conventional crude oil refinery, aims towards the complete utilization of the biomass through selective and cascade extraction of different components. It is important to note that in this scenario, operating conditions should be mild and not harmful to any of the products. A potential route would be to separate the protein and the lipid fraction first by taking advantage of the natural extruding of the protein in water after a mild pretreatment method such as microwaves. Lipid and pigment extraction can take place then with the addition of solvents. The residual biomass can then be further treated either with anaerobic digestion for production of methane or with high thermal liquefaction (HTL) for biocrude oil.
Bibliography
Explore our content and expand your knowledge.
2021
Radin Maya Saphira Radin Mohamed Maizatul Azrina Yaakob, Adel Al-Gheethi; Ambati, Ranga Rao
Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview Journal Article
In: Cells, vol. 10, iss. 2, pp. 393, 2021.
@article{nokey,
title = {Influence of Nitrogen and Phosphorus on Microalgal Growth, Biomass, Lipid, and Fatty Acid Production: An Overview},
author = {Maizatul Azrina Yaakob, Radin Maya Saphira Radin Mohamed, Adel Al-Gheethi, Ravishankar Aswathnarayana Gokare and Ranga Rao Ambati},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7918059/},
doi = {10.3390/cells10020393},
year = {2021},
date = {2021-02-14},
urldate = {2021-02-14},
journal = {Cells},
volume = {10},
issue = {2},
pages = {393},
abstract = {Microalgae can be used as a source of alternative food, animal feed, biofuel, fertilizer, cosmetics, nutraceuticals and for pharmaceutical purposes. The extraction of organic constituents from microalgae cultivated in the different nutrient compositions is influenced by microalgal growth rates, biomass yield and nutritional content in terms of lipid and fatty acid production. In this context, nutrient composition plays an important role in microalgae cultivation, and depletion and excessive sources of this nutrient might affect the quality of biomass. Investigation on the role of nitrogen and phosphorus, which are crucial for the growth of algae, has been addressed. However, there are challenges for enhancing nutrient utilization efficiently for large scale microalgae cultivation. Hence, this study aims to highlight the level of nitrogen and phosphorus required for microalgae cultivation and focuses on the benefits of nitrogen and phosphorus for increasing biomass productivity of microalgae for improved lipid and fatty acid quantities. Furthermore, the suitable extraction methods that can be used to utilize lipid and fatty acids from microalgae for biofuel have also been reviewed.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Microalgae can be used as a source of alternative food, animal feed, biofuel, fertilizer, cosmetics, nutraceuticals and for pharmaceutical purposes. The extraction of organic constituents from microalgae cultivated in the different nutrient compositions is influenced by microalgal growth rates, biomass yield and nutritional content in terms of lipid and fatty acid production. In this context, nutrient composition plays an important role in microalgae cultivation, and depletion and excessive sources of this nutrient might affect the quality of biomass. Investigation on the role of nitrogen and phosphorus, which are crucial for the growth of algae, has been addressed. However, there are challenges for enhancing nutrient utilization efficiently for large scale microalgae cultivation. Hence, this study aims to highlight the level of nitrogen and phosphorus required for microalgae cultivation and focuses on the benefits of nitrogen and phosphorus for increasing biomass productivity of microalgae for improved lipid and fatty acid quantities. Furthermore, the suitable extraction methods that can be used to utilize lipid and fatty acids from microalgae for biofuel have also been reviewed.
Maltsev, Yevhen; Maltseva, Kateryna; Kulikovskiy, Maxim; Maltseva, Svetlana
Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition Journal Article
In: Biology, vol. 10, no. 10, 2021, ISSN: 2079-7737.
@article{biology10101060,
title = {Influence of Light Conditions on Microalgae Growth and Content of Lipids, Carotenoids, and Fatty Acid Composition},
author = {Yevhen Maltsev and Kateryna Maltseva and Maxim Kulikovskiy and Svetlana Maltseva},
url = {https://www.mdpi.com/2079-7737/10/10/1060},
doi = {10.3390/biology10101060},
issn = {2079-7737},
year = {2021},
date = {2021-01-01},
urldate = {2021-01-01},
journal = {Biology},
volume = {10},
number = {10},
abstract = {Microalgae are a valuable natural resource for a variety of value-added products. The growth of microalgae is determined by the impact of many factors, but, from the point of view of the implementation of autotrophic growth, light is of primary importance. This work presents an overview of the influence of light conditions on the growth of microalgae, the content of lipids, carotenoids, and the composition of fatty acids in their biomass, taking into account parameters such as the intensity, duration of lighting, and use of rays of different spectral composition. The optimal light intensity for the growth of microalgae lies in the following range: 26−400 µmol photons m−2 s−1. An increase in light intensity leads to an activation of lipid synthesis. For maximum lipid productivity, various microalgae species and strains need lighting of different intensities: from 60 to 700 µmol photons m−2 s−1. Strong light preferentially increases the triacylglyceride content. The intensity of lighting has a regulating effect on the synthesis of fatty acids, carotenoids, including β-carotene, lutein and astaxanthin. In intense lighting conditions, saturated fatty acids usually accumulate, as well as monounsaturated ones, and the number of polyunsaturated fatty acids decreases. Red as well as blue LED lighting improves the biomass productivity of microalgae of various taxonomic groups. Changing the duration of the photoperiod, the use of pulsed light can stimulate microalgae growth, the production of lipids, and carotenoids. The simultaneous use of light and other stresses contributes to a stronger effect on the productivity of algae.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Microalgae are a valuable natural resource for a variety of value-added products. The growth of microalgae is determined by the impact of many factors, but, from the point of view of the implementation of autotrophic growth, light is of primary importance. This work presents an overview of the influence of light conditions on the growth of microalgae, the content of lipids, carotenoids, and the composition of fatty acids in their biomass, taking into account parameters such as the intensity, duration of lighting, and use of rays of different spectral composition. The optimal light intensity for the growth of microalgae lies in the following range: 26−400 µmol photons m−2 s−1. An increase in light intensity leads to an activation of lipid synthesis. For maximum lipid productivity, various microalgae species and strains need lighting of different intensities: from 60 to 700 µmol photons m−2 s−1. Strong light preferentially increases the triacylglyceride content. The intensity of lighting has a regulating effect on the synthesis of fatty acids, carotenoids, including β-carotene, lutein and astaxanthin. In intense lighting conditions, saturated fatty acids usually accumulate, as well as monounsaturated ones, and the number of polyunsaturated fatty acids decreases. Red as well as blue LED lighting improves the biomass productivity of microalgae of various taxonomic groups. Changing the duration of the photoperiod, the use of pulsed light can stimulate microalgae growth, the production of lipids, and carotenoids. The simultaneous use of light and other stresses contributes to a stronger effect on the productivity of algae.
2019
Gabriela F. Ferreira Sérgio S. de Jesus, Larissa S. Moreira
Comparison of several methods for effective lipid extraction from wet microalgae using green solvents Journal Article
In: Renewable Energy, vol. 143, pp. 130-141, 2019, ISSN: 0960-1481.
@article{nokey,
title = {Comparison of several methods for effective lipid extraction from wet microalgae using green solvents},
author = {Sérgio S. de Jesus, Gabriela F. Ferreira, Larissa S. Moreira, Maria Regina Wolf Maciel, Rubens Maciel Filho,},
doi = {https://doi.org/10.1016/j.renene.2019.04.168},
issn = {0960-1481},
year = {2019},
date = {2019-12-01},
urldate = {2019-12-01},
journal = {Renewable Energy},
volume = {143},
pages = {130-141},
abstract = {A comparative study of lipid extraction from microalgae was performed using the Soxhlet, Bligh and Dyer, Folch, and Hara and Radin methods, with the green solvents 2-methyltetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME), which have also been used in previously published studies. Extractions were performed with the microalgae Chlorella pyrenoidosa at 65.71% moisture. The Bligh and Dyer methodology, using the solvents 2-MeTHF:isoamyl alcohol (2:1 v/v) and CPME:methanol (1:1.7 v/v), extracted 95.73 ± 0.52 and 89.35 ± 7.98 mg lipids/g biomass, respectively. Regarding fatty acids yield, CPME showed higher selectivity than 2-MeTHF. A brief cost-effectiveness and energy analysis of the extraction process was performed. Based on the calculations, this study concluded that the energy required for the evaporation of the solvent and mixture of solvents after the extraction process has no significant economic impact; the largest expense is associated with solvent consumption. To extract 1 kg of fatty acids, the Hara and Radin method using hexane:isopropanol (3:2 v/v) proved to be the most cost-effective. Results show that these solvents prices’ are still not competitive when compared to fossil-based solvents. The price reduction of 2-MeTHF would make it more attractive than CPME, as it requires a lower amount of biomass.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
A comparative study of lipid extraction from microalgae was performed using the Soxhlet, Bligh and Dyer, Folch, and Hara and Radin methods, with the green solvents 2-methyltetrahydrofuran (2-MeTHF) and cyclopentyl methyl ether (CPME), which have also been used in previously published studies. Extractions were performed with the microalgae Chlorella pyrenoidosa at 65.71% moisture. The Bligh and Dyer methodology, using the solvents 2-MeTHF:isoamyl alcohol (2:1 v/v) and CPME:methanol (1:1.7 v/v), extracted 95.73 ± 0.52 and 89.35 ± 7.98 mg lipids/g biomass, respectively. Regarding fatty acids yield, CPME showed higher selectivity than 2-MeTHF. A brief cost-effectiveness and energy analysis of the extraction process was performed. Based on the calculations, this study concluded that the energy required for the evaporation of the solvent and mixture of solvents after the extraction process has no significant economic impact; the largest expense is associated with solvent consumption. To extract 1 kg of fatty acids, the Hara and Radin method using hexane:isopropanol (3:2 v/v) proved to be the most cost-effective. Results show that these solvents prices’ are still not competitive when compared to fossil-based solvents. The price reduction of 2-MeTHF would make it more attractive than CPME, as it requires a lower amount of biomass.
In Yung Sunwoo So Hee Kim, Hee Jun Hong
In: Bioprocess and Biosystems Engineering, vol. 42, pp. 1517–1526, 2019.
@article{nokey,
title = {Lipid and unsaturated fatty acid productions from three microalgae using nitrate and light-emitting diodes with complementary LED wavelength in a two-phase culture system},
author = {So Hee Kim, In Yung Sunwoo, Hee Jun Hong, Che Clovis Awah, Gwi-Taek Jeong & Sung-Koo Kim},
url = {https://link.springer.com/article/10.1007/s00449-019-02149-y#article-info},
doi = {https://doi.org/10.1007/s00449-019-02149-y},
year = {2019},
date = {2019-05-20},
journal = {Bioprocess and Biosystems Engineering},
volume = {42},
pages = {1517–1526},
abstract = {In this study, Pavlova lutheri, Chlorella vulgaris, and Porphyridium cruentum were cultured using modified F/2 media in a 1 L flask culture. Various nitrate concentrations were tested to determine an optimal nitrate concentration for algal growth. Subsequently, the effect of light emitted at a specific wavelength on biomass and lipid production by three microalgae was evaluated using various wavelengths of light-emitting diodes (LED). Biomass production by P. lutheri, C. vulgaris, and P. cruentum were the highest with blue, red, and green LED wavelength with 1.09 g dcw/L, 1.23 g dcw/L, and 1.28 g dcw/L on day 14, respectively. Biomass production was highest at the complementary LED wavelength to the color of microalgae. Lipid production by P. lutheri, C. vulgaris, and P. cruentum were the highest with yellow, green, and red LEDs’ wavelength, respectively. Eicosapentaenoic acid production by P. lutheri, C. vulgaris, and P. cruentum was 10.35%, 10.14%, and 14.61%, and those of docosahexaenoic acid were 6.09%, 8.95%, and 11.29%, respectively.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
In this study, Pavlova lutheri, Chlorella vulgaris, and Porphyridium cruentum were cultured using modified F/2 media in a 1 L flask culture. Various nitrate concentrations were tested to determine an optimal nitrate concentration for algal growth. Subsequently, the effect of light emitted at a specific wavelength on biomass and lipid production by three microalgae was evaluated using various wavelengths of light-emitting diodes (LED). Biomass production by P. lutheri, C. vulgaris, and P. cruentum were the highest with blue, red, and green LED wavelength with 1.09 g dcw/L, 1.23 g dcw/L, and 1.28 g dcw/L on day 14, respectively. Biomass production was highest at the complementary LED wavelength to the color of microalgae. Lipid production by P. lutheri, C. vulgaris, and P. cruentum were the highest with yellow, green, and red LEDs’ wavelength, respectively. Eicosapentaenoic acid production by P. lutheri, C. vulgaris, and P. cruentum was 10.35%, 10.14%, and 14.61%, and those of docosahexaenoic acid were 6.09%, 8.95%, and 11.29%, respectively.
2018
Demory, David; Combe, Charlotte; Hartmann, Philipp; Talec, Amélie; Pruvost, Eric; Hamouda, Raouf; Souillé, Fabien; Lamare, Pierre-Olivier; Bristeau, Marie-Odile; Sainte-Marie, Jacques; Rabouille, Sophie; Mairet, Francis; Sciandra, Antoine; Bernard, Olivier
How do microalgae perceive light in a high-rate pond? Towards more realistic Lagrangian experiments Journal Article
In: Royal Society Open Science, vol. 5, no. 180523, 2018, ISSN: 2054-5703.
@article{Demory2018,
title = {How do microalgae perceive light in a high-rate pond? Towards more realistic Lagrangian experiments},
author = {David Demory and Charlotte Combe and Philipp Hartmann and Amélie Talec and Eric Pruvost and Raouf Hamouda and Fabien Souillé and Pierre-Olivier Lamare and Marie-Odile Bristeau and Jacques Sainte-Marie and Sophie Rabouille and Francis Mairet and Antoine Sciandra and Olivier Bernard},
doi = {10.1098/rsos.180523},
issn = {2054-5703},
year = {2018},
date = {2018-05-30},
urldate = {2018-05-00},
journal = {Royal Society Open Science},
volume = {5},
number = {180523},
publisher = {The Royal Society},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Charlotte Combe David Demory, Philipp Hartmann; Bernard, Olivier
How do microalgae perceive light in a high-rate pond? Towards more realistic Lagrangian experiments Journal Article
In: Royal Society, vol. 5, iss. 5, 2018, ISSN: 2054-5703.
@article{nokey,
title = {How do microalgae perceive light in a high-rate pond? Towards more realistic Lagrangian experiments},
author = {David Demory, Charlotte Combe, Philipp Hartmann, Amélie Talec, Eric Pruvost, Raouf Hamouda, Fabien Souillé, Pierre-Olivier Lamare, Marie-Odile Bristeau, Jacques Sainte-Marie, Sophie Rabouille, Francis Mairet, Antoine Sciandra and Olivier Bernard},
url = {https://royalsocietypublishing.org/doi/10.1098/rsos.180523},
doi = {https://doi.org/10.1098/rsos.180523},
issn = {2054-5703},
year = {2018},
date = {2018-05-30},
journal = {Royal Society},
volume = {5},
issue = {5},
abstract = {Hydrodynamics in a high-rate production reactor for microalgae cultivation affects the light history perceived by cells. The interplay between cell movement and medium turbidity leads to a complex light pattern, whose forcing effects on photosynthesis and photoacclimation dynamics are non-trivial. Hydrodynamics of high density algal ponds mixed by a paddle wheel has been studied recently, although the focus has never been on describing its impact on photosynthetic growth efficiency. In this multidisciplinary downscaling study, we first reconstructed single cell trajectories in an open raceway using an original hydrodynamical model offering a powerful discretization of the Navier–Stokes equations tailored to systems with free surfaces. The trajectory of a particular cell was selected and the associated high-frequency light pattern was computed. This light pattern was then experimentally reproduced in an Arduino-driven computer controlled cultivation system with a low density Dunaliella salina culture. The effect on growth and pigment content was recorded for various frequencies of the light pattern, by setting different paddle wheel velocities. Results show that the frequency of this realistic signal plays a decisive role in the dynamics of photosynthesis, thus revealing an unexpected photosynthetic response compared to that recorded under the on/off signals usually used in the literature. Indeed, the light received by a single cell contains signals from low to high frequencies that nonlinearly interact with the photosynthesis process and differentially stimulate the various time scales associated with photoacclimation and energy dissipation. This study highlights the need for experiments with more realistic light stimuli to better understand microalgal growth at high cell densities. An experimental protocol is also proposed, with simple, yet more realistic, step functions for light fluctuations.
},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Hydrodynamics in a high-rate production reactor for microalgae cultivation affects the light history perceived by cells. The interplay between cell movement and medium turbidity leads to a complex light pattern, whose forcing effects on photosynthesis and photoacclimation dynamics are non-trivial. Hydrodynamics of high density algal ponds mixed by a paddle wheel has been studied recently, although the focus has never been on describing its impact on photosynthetic growth efficiency. In this multidisciplinary downscaling study, we first reconstructed single cell trajectories in an open raceway using an original hydrodynamical model offering a powerful discretization of the Navier–Stokes equations tailored to systems with free surfaces. The trajectory of a particular cell was selected and the associated high-frequency light pattern was computed. This light pattern was then experimentally reproduced in an Arduino-driven computer controlled cultivation system with a low density Dunaliella salina culture. The effect on growth and pigment content was recorded for various frequencies of the light pattern, by setting different paddle wheel velocities. Results show that the frequency of this realistic signal plays a decisive role in the dynamics of photosynthesis, thus revealing an unexpected photosynthetic response compared to that recorded under the on/off signals usually used in the literature. Indeed, the light received by a single cell contains signals from low to high frequencies that nonlinearly interact with the photosynthesis process and differentially stimulate the various time scales associated with photoacclimation and energy dissipation. This study highlights the need for experiments with more realistic light stimuli to better understand microalgal growth at high cell densities. An experimental protocol is also proposed, with simple, yet more realistic, step functions for light fluctuations.
Xu, Yanan; Ibrahim, Iskander M.; Wosu, Chiziezi I.; Ben-Amotz, Ami; Harvey, Patricia J.
Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production Journal Article
In: Biology, vol. 7, no. 1, 2018, ISSN: 2079-7737.
@article{biology7010014,
title = {Potential of New Isolates of Dunaliella Salina for Natural β-Carotene Production},
author = {Yanan Xu and Iskander M. Ibrahim and Chiziezi I. Wosu and Ami Ben-Amotz and Patricia J. Harvey},
url = {https://www.mdpi.com/2079-7737/7/1/14},
doi = {10.3390/biology7010014},
issn = {2079-7737},
year = {2018},
date = {2018-01-01},
urldate = {2018-01-01},
journal = {Biology},
volume = {7},
number = {1},
abstract = {The halotolerant microalga Dunaliella salina has been widely studied for natural β-carotene production. This work shows biochemical characterization of three newly isolated Dunaliella salina strains, DF15, DF17, and DF40, compared with D. salina CCAP 19/30 and D. salina UTEX 2538 (also known as D. bardawil). Although all three new strains have been genetically characterized as Dunaliella salina strains, their ability to accumulate carotenoids and their capacity for photoprotection against high light stress are different. DF15 and UTEX 2538 reveal great potential for producing a large amount of β-carotene and maintained a high rate of photosynthesis under light of high intensity; however, DF17, DF40, and CCAP 19/30 showed increasing photoinhibition with increasing light intensity, and reduced contents of carotenoids, in particular β-carotene, suggesting that the capacity of photoprotection is dependent on the cellular content of carotenoids, in particular β-carotene. Strong positive correlations were found between the cellular content of all-trans β-carotene, 9-cis β-carotene, all-trans α-carotene and zeaxanthin but not lutein in the D. salina strains. Lutein was strongly correlated with respiration in photosynthetic cells and strongly related to photosynthesis, chlorophyll and respiration, suggesting an important and not hitherto identified role for lutein in coordinated control of the cellular functions of photosynthesis and respiration in response to changes in light conditions, which is broadly conserved in Dunaliella strains. Statistical analysis based on biochemical data revealed a different grouping strategy from the genetic classification of the strains. The significance of these data for strain selection for commercial carotenoid production is discussed.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The halotolerant microalga Dunaliella salina has been widely studied for natural β-carotene production. This work shows biochemical characterization of three newly isolated Dunaliella salina strains, DF15, DF17, and DF40, compared with D. salina CCAP 19/30 and D. salina UTEX 2538 (also known as D. bardawil). Although all three new strains have been genetically characterized as Dunaliella salina strains, their ability to accumulate carotenoids and their capacity for photoprotection against high light stress are different. DF15 and UTEX 2538 reveal great potential for producing a large amount of β-carotene and maintained a high rate of photosynthesis under light of high intensity; however, DF17, DF40, and CCAP 19/30 showed increasing photoinhibition with increasing light intensity, and reduced contents of carotenoids, in particular β-carotene, suggesting that the capacity of photoprotection is dependent on the cellular content of carotenoids, in particular β-carotene. Strong positive correlations were found between the cellular content of all-trans β-carotene, 9-cis β-carotene, all-trans α-carotene and zeaxanthin but not lutein in the D. salina strains. Lutein was strongly correlated with respiration in photosynthetic cells and strongly related to photosynthesis, chlorophyll and respiration, suggesting an important and not hitherto identified role for lutein in coordinated control of the cellular functions of photosynthesis and respiration in response to changes in light conditions, which is broadly conserved in Dunaliella strains. Statistical analysis based on biochemical data revealed a different grouping strategy from the genetic classification of the strains. The significance of these data for strain selection for commercial carotenoid production is discussed.
2017
Bleakley, Stephen; Hayes, Maria
Algal Proteins: Extraction, Application, and Challenges Concerning Production Journal Article
In: Foods, vol. 6, iss. 5, pp. 33, 2017.
@article{nokey,
title = {Algal Proteins: Extraction, Application, and Challenges Concerning Production},
author = {Stephen Bleakley and Maria Hayes},
editor = {Population growth combined with increasingly limited resources of arable land and fresh water has resulted in a need for alternative protein sources. Macroalgae (seaweed) and microalgae are examples of under-exploited “crops”. Algae do not compete with traditional food crops for space and resources. This review details the characteristics of commonly consumed algae, as well as their potential for use as a protein source based on their protein quality, amino acid composition, and digestibility. Protein extraction methods applied to algae to date, including enzymatic hydrolysis, physical processes, and chemical extraction and novel methods such as ultrasound-assisted extraction, pulsed electric field, and microwave-assisted extraction are discussed. Moreover, existing protein enrichment methods used in the dairy industry and the potential of these methods to generate high value ingredients from algae, such as bioactive peptides and functional ingredients are discussed. Applications of algae in human nutrition, animal feed, and aquaculture are examined.},
doi = {10.3390/foods6050033},
year = {2017},
date = {2017-04-26},
urldate = {2017-04-26},
journal = {Foods},
volume = {6},
issue = {5},
pages = {33},
abstract = {Population growth combined with increasingly limited resources of arable land and fresh water has resulted in a need for alternative protein sources. Macroalgae (seaweed) and microalgae are examples of under-exploited “crops”. Algae do not compete with traditional food crops for space and resources. This review details the characteristics of commonly consumed algae, as well as their potential for use as a protein source based on their protein quality, amino acid composition, and digestibility. Protein extraction methods applied to algae to date, including enzymatic hydrolysis, physical processes, and chemical extraction and novel methods such as ultrasound-assisted extraction, pulsed electric field, and microwave-assisted extraction are discussed. Moreover, existing protein enrichment methods used in the dairy industry and the potential of these methods to generate high value ingredients from algae, such as bioactive peptides and functional ingredients are discussed. Applications of algae in human nutrition, animal feed, and aquaculture are examined.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Population growth combined with increasingly limited resources of arable land and fresh water has resulted in a need for alternative protein sources. Macroalgae (seaweed) and microalgae are examples of under-exploited “crops”. Algae do not compete with traditional food crops for space and resources. This review details the characteristics of commonly consumed algae, as well as their potential for use as a protein source based on their protein quality, amino acid composition, and digestibility. Protein extraction methods applied to algae to date, including enzymatic hydrolysis, physical processes, and chemical extraction and novel methods such as ultrasound-assisted extraction, pulsed electric field, and microwave-assisted extraction are discussed. Moreover, existing protein enrichment methods used in the dairy industry and the potential of these methods to generate high value ingredients from algae, such as bioactive peptides and functional ingredients are discussed. Applications of algae in human nutrition, animal feed, and aquaculture are examined.
2015
imagePolur Hanumantha Rao Ramanathan Ranjith Kumar, imageMuthu Arumugam
Lipid extraction methods from microalgae: a comprehensive review Journal Article
In: Frontiers in Energy Research, vol. 2, 2015.
@article{nokey,
title = {Lipid extraction methods from microalgae: a comprehensive review},
author = {Ramanathan Ranjith Kumar, imagePolur Hanumantha Rao, imageMuthu Arumugam},
url = {https://www.frontiersin.org/articles/10.3389/fenrg.2014.00061/full},
doi = { https://doi.org/10.3389/fenrg.2014.00061},
year = {2015},
date = {2015-01-08},
urldate = {2015-01-08},
journal = {Frontiers in Energy Research},
volume = {2},
abstract = {Energy security has become a serious global issue and a lot of research is being carried out to look for economically viable and environment-friendly alternatives. The only solution that appears to meet futuristic needs is the use of renewable energy. Although various forms of renewable energy are being currently used, the prospects of producing carbon-neutral biofuels from microalgae appear bright because of their unique features such as suitability of growing in open ponds required for production of a commodity product, high CO2-sequestering capability, and ability to grow in wastewater/seawater/brackish water and high-lipid productivity. The major process constraint in microalgal biofuel technology is the cost-effective and efficient extraction of lipids. The objective of this article is to provide a comprehensive review on various methods of lipid extraction from microalgae available, to date, as well as to discuss their advantages and disadvantages. The article covers all areas of lipid extraction procedures including solvent extraction procedures, mechanical approaches, and solvent-free procedures apart from some of the latest extraction technologies. Further research is required in this area for successful implementation of this technology at the production scale.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Energy security has become a serious global issue and a lot of research is being carried out to look for economically viable and environment-friendly alternatives. The only solution that appears to meet futuristic needs is the use of renewable energy. Although various forms of renewable energy are being currently used, the prospects of producing carbon-neutral biofuels from microalgae appear bright because of their unique features such as suitability of growing in open ponds required for production of a commodity product, high CO2-sequestering capability, and ability to grow in wastewater/seawater/brackish water and high-lipid productivity. The major process constraint in microalgal biofuel technology is the cost-effective and efficient extraction of lipids. The objective of this article is to provide a comprehensive review on various methods of lipid extraction from microalgae available, to date, as well as to discuss their advantages and disadvantages. The article covers all areas of lipid extraction procedures including solvent extraction procedures, mechanical approaches, and solvent-free procedures apart from some of the latest extraction technologies. Further research is required in this area for successful implementation of this technology at the production scale.
Suh, William I.; Mishra, Sanjiv K.; Kim, Tae-Hyoung; Farooq, Wasif; Moon, Myounghoon; Shrivastav, Anupama; Park, Min S.; Yang, Ji-Won
Direct transesterification of wet microalgal biomass for preparation of biodiesel Journal Article
In: Algal Research, vol. 12, pp. 405-411, 2015, ISSN: 2211-9264.
@article{SUH2015405,
title = {Direct transesterification of wet microalgal biomass for preparation of biodiesel},
author = {William I. Suh and Sanjiv K. Mishra and Tae-Hyoung Kim and Wasif Farooq and Myounghoon Moon and Anupama Shrivastav and Min S. Park and Ji-Won Yang},
url = {https://www.sciencedirect.com/science/article/pii/S2211926415300801},
doi = {https://doi.org/10.1016/j.algal.2015.10.006},
issn = {2211-9264},
year = {2015},
date = {2015-01-01},
urldate = {2015-01-01},
journal = {Algal Research},
volume = {12},
pages = {405-411},
abstract = {Most conventional processes for algal biodiesel production involve separate lipid extraction steps or require usage of dry biomass that incurs extra cost and an energy intensive drying step. A novel process that involves dehydration of wet biomass via pretreatment with ethanol followed by direct in situ transesterification into biodiesel was investigated in this study. Under mild esterification at 80°C for 30min, pretreating the wet biomass twice with 3 volumes of ethanol resulted in a nearly four-fold increase of fatty acid ethyl ester (FAEE) yield from 3.04mg to 11.78mg, while increasing the ethanol from 1 volume to 10 volumes resulted in a six fold increase of yield from 3.18 to 18.29mg. The FAEE yield further increased when the esterification reaction was run at higher temperature and longer durations of up to 120°C for 2h. The overall positive impact of the pretreatment step on the final yield was far greater for milder reaction conditions, which makes the process more attractive in terms of economics and energy savings. In addition, it was found that the yield is unaffected by the choice of alcohol, which means methanol and butanol can also be used for the process. Lastly, it was found that the low concentration of water in the FAEE containing spent ethanol meant that both the solvent and sulfuric acid could be reused to further concentrate the quantity of FAEE in the final product mixture.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Most conventional processes for algal biodiesel production involve separate lipid extraction steps or require usage of dry biomass that incurs extra cost and an energy intensive drying step. A novel process that involves dehydration of wet biomass via pretreatment with ethanol followed by direct in situ transesterification into biodiesel was investigated in this study. Under mild esterification at 80°C for 30min, pretreating the wet biomass twice with 3 volumes of ethanol resulted in a nearly four-fold increase of fatty acid ethyl ester (FAEE) yield from 3.04mg to 11.78mg, while increasing the ethanol from 1 volume to 10 volumes resulted in a six fold increase of yield from 3.18 to 18.29mg. The FAEE yield further increased when the esterification reaction was run at higher temperature and longer durations of up to 120°C for 2h. The overall positive impact of the pretreatment step on the final yield was far greater for milder reaction conditions, which makes the process more attractive in terms of economics and energy savings. In addition, it was found that the yield is unaffected by the choice of alcohol, which means methanol and butanol can also be used for the process. Lastly, it was found that the low concentration of water in the FAEE containing spent ethanol meant that both the solvent and sulfuric acid could be reused to further concentrate the quantity of FAEE in the final product mixture.
2010
Sayre, Richard
Microalgae: The Potential for Carbon Capture Journal Article
In: BioScience, vol. 60, no. 9, pp. 722-727, 2010, ISSN: 0006-3568.
@article{10.1525/bio.2010.60.9.9,
title = {Microalgae: The Potential for Carbon Capture},
author = {Richard Sayre},
url = {https://doi.org/10.1525/bio.2010.60.9.9},
doi = {10.1525/bio.2010.60.9.9},
issn = {0006-3568},
year = {2010},
date = {2010-01-01},
urldate = {2010-01-01},
journal = {BioScience},
volume = {60},
number = {9},
pages = {722-727},
abstract = {There is growing recognition that microalgae are among the most productive biological systems for generating biomass and capturing carbon. Further efficiencies are gained by harvesting 100% of the biomass, much more than is possible in terrestrial biomass production systems. Micro-algae's ability to transport bicarbonate into cells makes them well suited to capture carbon. Carbon dioxide—or bicarbonate-capturing efficiencies as high as 90% have been reported in open ponds. The scale of microalgal production facilities necessary to capture carbon-dioxide (CO2) emissions from stationary point sources such as power stations and cement kilns is also manageable; thus, microalgae can potentially be exploited for CO2 capture and sequestration. In this article, I discuss possible strategies using microalgae to sequester CO2 with reduced environmental consequences.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
There is growing recognition that microalgae are among the most productive biological systems for generating biomass and capturing carbon. Further efficiencies are gained by harvesting 100% of the biomass, much more than is possible in terrestrial biomass production systems. Micro-algae's ability to transport bicarbonate into cells makes them well suited to capture carbon. Carbon dioxide—or bicarbonate-capturing efficiencies as high as 90% have been reported in open ponds. The scale of microalgal production facilities necessary to capture carbon-dioxide (CO2) emissions from stationary point sources such as power stations and cement kilns is also manageable; thus, microalgae can potentially be exploited for CO2 capture and sequestration. In this article, I discuss possible strategies using microalgae to sequester CO2 with reduced environmental consequences.
2009
Jacob-Lopes, Eduardo; Scoparo, Carlos Henrique Gimenes; Lacerda, Lucy Mara Cacia Ferreira; Franco, Telma Teixeira
Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors Journal Article
In: Chemical Engineering and Processing: Process Intensification, vol. 48, no. 1, pp. 306-310, 2009, ISSN: 0255-2701.
@article{JACOBLOPES2009306,
title = {Effect of light cycles (night/day) on CO2 fixation and biomass production by microalgae in photobioreactors},
author = {Eduardo Jacob-Lopes and Carlos Henrique Gimenes Scoparo and Lucy Mara Cacia Ferreira Lacerda and Telma Teixeira Franco},
url = {https://www.sciencedirect.com/science/article/pii/S0255270108001037},
doi = {https://doi.org/10.1016/j.cep.2008.04.007},
issn = {0255-2701},
year = {2009},
date = {2009-01-01},
urldate = {2009-01-01},
journal = {Chemical Engineering and Processing: Process Intensification},
volume = {48},
number = {1},
pages = {306-310},
abstract = {The objective of this study was to evaluate the effect of the photoperiod on the biomass production and carbon dioxide fixation rates using a photosynthetic culture of the cyanobacterium Aphanothece microscopica Nägeli in bubble column photobioreactors. The cultures were carried out at temperatures of 35°C, air enriched with carbon dioxide at concentrations of 15% and photon flux density of 150μmolm−2s−1. The light cycles evaluated were 0:24, 2:22, 4:20, 6:18, 8:16, 10:14, 12:12, 14:10, 16:8, 18:6, 20:4, 22:2 and 24:0 (night:day), respectively. The results obtained indicated that the duration of the light periods was a determinant factor in the performance of the photobioreactors. A linear reduction in biomass production and carbon dioxide fixation with reductions in the duration of the light period was evident, with the exception of the 12:12 (night:day) cycles. Reductions of up to 99.69% in the carbon-fixation rates as compared with cultures under continuous illumination were obtained.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
The objective of this study was to evaluate the effect of the photoperiod on the biomass production and carbon dioxide fixation rates using a photosynthetic culture of the cyanobacterium Aphanothece microscopica Nägeli in bubble column photobioreactors. The cultures were carried out at temperatures of 35°C, air enriched with carbon dioxide at concentrations of 15% and photon flux density of 150μmolm−2s−1. The light cycles evaluated were 0:24, 2:22, 4:20, 6:18, 8:16, 10:14, 12:12, 14:10, 16:8, 18:6, 20:4, 22:2 and 24:0 (night:day), respectively. The results obtained indicated that the duration of the light periods was a determinant factor in the performance of the photobioreactors. A linear reduction in biomass production and carbon dioxide fixation with reductions in the duration of the light period was evident, with the exception of the 12:12 (night:day) cycles. Reductions of up to 99.69% in the carbon-fixation rates as compared with cultures under continuous illumination were obtained.
1956
K. A. Gilles Michel DuBois, J. K. Hamilton; Smith, Fred.
Colorimetric Method for Determination of Sugars and Related Substances Journal Article
In: Analytical Chemistry, vol. 28, iss. 3, pp. 350-356, 1956.
@article{nokey,
title = {Colorimetric Method for Determination of Sugars and Related Substances},
author = {Michel DuBois, K. A. Gilles, J. K. Hamilton, P. A. Rebers, and Fred. Smith},
doi = {https://doi.org/10.1021/ac60111a017},
year = {1956},
date = {1956-03-01},
urldate = {1956-03-01},
journal = {Analytical Chemistry},
volume = {28},
issue = {3},
pages = {350-356},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Feel free to navigate through the different parts of the BlueBioChain NextGen training programme, following the hierarchy of the structure outlined below.
Click “Learn more” to dive into the next layer of the hierarchy.

The project “Novel biorefinery supply chains for wastewater valorization and production of high market value bio products using microalgae (BlueBioChain)” Project ID 31, is part of the BlueBio ERA-NET Cofund under the European Union’s Horizon 2020 Research and Innovation programme (Grant agreement No. 817992) (Project ID 31 BlueBioChain).

Copyright 2022. All Rights Reserved.
info@bluebiochain.eu